Пошкодження периферичних нервів кінцівки при механічно-індукованій ішемії
1 Підлісецкий А.Т., 2 Савосько С.І., 3 Долгополов О.В., 4 Макаренко А.Н.
1 Львівський обласний госпіталь ветеранів воєн та репресованих ім. Ю. Ліпи;
2 Національний медичний університет ім. О. О. Богомольця, кафедра гістології та ембріології, Київ;
3 ДУ «Інститут ортопедії та травматології НАМН України», Київ;
4 Міжрегіональна Академія управління персоналом, Київ.
У статті описано результати дослідження змін периферичних нервів кінцівки після механічно індукованої ішемії. Оцінено мієлінові нервові волокна в сідничному та великогомілковому нерві після 6-годинної ішемії, модельованої джгутом на рівні гомілки, колінного суглоба та нижньої третини стегна. У фасцикулах сідничного нерва змін щільності нервових волокон на 5, 15 та 30 добу після моделювання ішемії не виявлено, проте виявлено деформовані волокна з різною товщиною мієлінової оболонки. У великогомілковому нерві на 5 та 15 добу встановлені демієлінізація та деформація мієліну, а на 15 та 30 добу – атрофія нервових волокон.
У нейролемоцитах пошкоджених мієлінових нервових волокон виявлено ознаки дистрофічних процесів та аутофагії. Після введення в ішемічно пошкоджені м’язи тромбоцитарної плазми, концентрату клітин аспірату кісткового мозку та гомогенізованої жирової тканини суттєвої різниці у ступеню пошкодження нерва не виявлено. Зроблено висновок, що структурні ознаки ушкодження периферичних нервів кінцівки після ішемії є неспецифічними і здебільшого залежать від рівня ушкодження кінцівки, ніж від терапевтичних підходів з використанням аутологічних клітинних технологій.
Peripheral nerve lesions after a mechanically induced limb ischemia
1 Pidlisetsky А., 2 Savosko S., 3 Dolgopolov О., 4 Makarenko O.
1 lviv regional Hospital for war Veterans and repressed named after Yu. lypa;
2 Bogomolets national Medical University, department of Histology and embryology;
3 SI “institute of Traumatology and orthopedics under NAMS of Ukraine;
4 Interregional academy of Personnel Management, Ukraine
Non-compression damage to peripheral nerves of a limb is not a frequent trauma to extremities. Non-compression ischemia relates to lesions of a limb’s great vessels (atherosclerosis, emboli, vasculitis, others). Frequently, they are consequences of a traumatic injury to an extremity [16]. A range of publications [17] contains selective descriptions of clinical cases of ischemic neuropathy, but the pathogenesis of damage to nerves of a limb remains understudied and unstructured. The reasons thereof are that the authors in their clinical researches describe separate cases with different etiology, while experimental studies made us able to investigate the development of damage to a limb’s nerves and their recovery. Their main disadvantage was the focus on just a single factor as a task – ischemia of a nerve, while clinical cases are complicated with the probable presence of two – ischemia itself and pressure on the nerve. Moreover, the experimental researches mostly limit themselves by one or two models of isolated nerve compression [8, 10]. Although, such cases rarely occur in clinical practice, and the majority of the compression lesions are the consequences of a simultaneous trauma and compression to a limb with significant damage to skeletal muscles followed by their atrophy, tissue necrosis.
Acute necrosis of skeletal muscles and consequences thereof (myoglobinuria, impaired metabolism of electrolytes, acute renal impairment) are covered by scholars better, compared to the same of nerves of limbs. The conditions of peripheral nerves in compression and non-compression ischemia could reflect all the tissues of the extremity and the efficiency of the therapy in general. Besides, the literature sources lack enough data on the extent of the damage to muscles and peripheral nerves of an extremity in compression injury, and, which is the most important, their sensitivity to damage and stimulation of repairing processes.
In traumatology, the autologous tissue technologies are widely used, in particular for managing arthritis, traumas to a limb’s nerves and tendons. Quite simple to obtain and use, and very promising are concentrates of platelet-rich plasma (PRP) and cells collected from the bone marrow aspirates (CBMA), and homogenized adipose tissue (HAT) derivable, especially in cases of peripheral neuropathies [10]. We found a range of researches with positive and promising results of the use thereof in the management of damages to the peripheral nervous system.
The technology seems useful for the therapy of compression ischemia neuropathies of the nerves in cases after a compartment syndrome.
Using experimental models of compression ischemia of a limb or peripheral nerves, we obtained a significant amount of data, but we should not equate all the results with the same of a human compression ischemia, so these disorders require further study.
The task of the research is to study damages to a sciatic nerve in mechanically-induced limb ischemia and after PRP, CMBA, and HAT injections.
Material and methods. The background of the research is the animal model, a mechanically induced ischemia (MII). The study involved Chinchilla rabbits (weighing 4.2-4.5 kg). The rabbits lived in standard cages with free access to food and water. We subdivided the animals into five groups, five animals each: group 1 (control) – intact animals; group 2 (MII) – animals with an injured limb; group 3 (MII+PRP) – animals with an injured limb treated by PRP; group 4 (МІІ+СBMA) – animals with an injured limb treated by СBMA; group 4 (МІІ+НАТ) – animals with an injured limb treated by НАТ.
MII was simulated by an elastic medical tourniquet (5.5 cm wide, eight units per an extremity), lasting through the middle third of a left hind limb, from the thigh to the ankle joint. It thereby immobilized the limb and caused impaired vascular perfusion of the skeletal muscles of the tibia (SMT). We removed the tourniquets after 6 hours.
The subfascial pressure. For the subfascial pressure measuring, we applied a classical invasive method whitesides in an anterolateral sleeve of a shin, using a standard device stryker intra-compartmental Pressure Monitor (USA) for a one-shot measurement of every value [2].
The cells suspensions preparation. The cell suspensions were injected into the upper third of the SMT once, 6 hours after the elastic tourniquet removal. Test animals got withdrawn for the experiment in 5th, 15th, and 30th days after it started with the limb MII (5 animals for each stage thereof).
The animals received anesthesia with 60 mg/kg sodium thiopental, intraperitoneal.
Platelet-rich plasma (PRP). We drew 5 ml of blood from the ear vein of a rabbit. By centrifuging the whole blood in an arthrex tube (Arthrex ACP® Double Syringe System, Arthrex, Inc. USA) for 8 minutes at 760g, we obtained 1 ml of PRP under the layer of erythrocyte mass (the average platelet quantity was 928,000/ml).
Autologous concentrated bone marrow aspirate (CBMA). We aspirated 2 mL of bone marrow from the proximal part of an intact femur, with a 10G bone puncture needle and 5 mL syringe, like in the article [1]. To separate the aspirate, we used a Tulip Emulsifier filter (Tulip Medical Products, USA). After centrifuging of the aspirate with dextrose citrate coagulate – solution A (1:8) (Baxter С.А., USA/Belgium) for 8 minutes at 760g, we got 1.0 mL of supraerythrocytic fraction (the average cell quantity in native aspirate – 55.800/ml).
Homogenized adipose tissue (HAT). Through the access 2 cm, we took 5 mg of an abdominal gland and fined it as a suspension. For homogenization, the said suspension passed through a 1mm hole of two connected syringes.
Histological study. Sciatic nerves and SMTs of rabbits were fixed in 10% formalin solution in PBS (pH 7.4) for 24 hours at 4° C. The 15 µm slices of sciatic nerves, obtained with a cryotome, we impregnated with silver nitrate. On longitudinal sections of the nerve, we calculated the number of nerve fibers in the test area (the transverse axis of the nerve – 420 µm, in the micrograph – 420×550 µm; 0.23 mm2).
Electron microscopy. The samples of tibial nerve were fixed in 2.5% solution of glutaraldehyde in phosphate buffer with 1% osmium tetrachloride, dehydrated in increasing concentrations of ethanol and acetone. The tissue samples we embedded in the Epone-Araldite mixture. To get the ultrathin slices, we applied an ultratome (reihart). For contrast, we used 2% sodium citrate and uranyl acetate. To analyze the sections obtained, we scanned them with an electron microscope Tescan Mira 3 lMU (CzechRepublic) (STEM detector). statistical analysis. The data obtained appeared as mean values (M) ± standard errors of the means (SEM) and have been compared using Mann-Whitney U-test. We took the differences as significant at P < 0.05.
Bioethics. All manipulations with the animals complied with the European Convention for the protection of vertebrate animals used for experimental and other scientific purposes, # 123, Council of Europe, L222, 24/08/1999, p. 31and the Commission on Bioethics of the State Institution “The Institute of Traumatology and Orthopedics under NAMS of Ukraine.”.
Result and discussion. Macroscopic studies. After the MII, limb muscles were specific with acute edema and aggregation of intestinal tissue. After the incision of the fascia, we registered the emission of 0.5 to 2.0 ml of the liquid. Large and medium subfascial vessels on the knee level and shin muscles (tibialis anterior, flexor digitorumlongus, gastrocremius) were visually full with excessive blood, with the areas of hemorrhagic leakages in muscle sections. The texture of shin muscles changed: the surface muscles were edematous, soft, decolorized (from pink to yellowish-white). The deep muscles were more elastic and pink. On the 15th day, the extent of the edema was lower, compared to the 5th day, and the tissue of the muscles – more elastic, while in 30th days after the experiment started, there was no excessive interstitial fluid anymore. The color of the muscle tissues changed towards pinky-yellow, yellowish-white. The group of animals treated with PRP and CBMA had no injection areas; in the group of HAT, the areas of the insertion of the suspension were clear, specific with the excessive formation of connective tissues and angiogenesis, evidencing encapsulation of the aspirate.
In all the animals with MII, we have detected the preservation of the integrity of sciatic nerves. The nerve was in the intramuscular space, without any signs of fibrosis processes along with it. The vessel network of the nerve and paraneural surroundings were distinct, the vessels full in blood, but without signs of hemorrhagic penetration into the intramuscular space. In other words, the mechanical damage to muscles of the shin, aggregation on interstitial fluid, and muscle tissue edema occur after the MII, decrease after the 5th day, and get replaced with necrotic changes in muscles without significant lesions to the sciatic nerve.
Microscopic changes in peripheral nerves of a limb. In longitudinal sections of nerves, we studied the structural changes of its fibers in the sciatic nerve fascicules. The nerve’s integrity was intact, the same for the fascicular structure. The sheaths of nerves had no reasonable damages, except for epineurium. We have noticed an increased density of fibroblasts, evidencing cellular reactions in the stromal elements of the nerve, cell proliferation in the epineurium. Nerve fibers were present in the nerve’s fascicules (Fig. 1), but their density had no significant differences within the period of the experiment (5, 15, and 30 days) and in terms of control. However, we have revealed deformed fibers with the different thickness of their myelin sheaths. On the 30th day after the MII, we identified “free” neurolemmocytes along the nerve fibers.
The distortion of a myelin sheath ultrastructure confirmed itself under electron microscopy (STEM). We studied the samples of the tibial nerve. On the 5th and 15th day, we noticed the impairment in the density of myelin lamellae, myelin sheath deformities, and necrotized fibers (Fig. 2). In the impaired myelin fibers, the axial cylinders could be intact (with microtubules, mitochondria, vesicles) or exfoliated from the myelin. The cytoplasm of neurolemmocytes contained atypical lamellar (membrane) structures, the primary pathologic feature of autophagy. Despite degeneration ovoids, appearing after the total disintegration of a myelin fiber, the cytosolic lamellar inclusions result from autophagy, alternation of the damaged myelin in neurolemmocytes, and are the signs of dystrophy [14]. The PRP, CBMA, and HAT groups had no significant difference in the extent of the damage to the nerve. All the samples studied confirmed the demyelination and necrosis. The changes in the myelin fibers were not specific and frequently diffused. Consequently, we concluded no dependence on the level of the damage in the separate fascicules.
We have not confirmed severe nerve fibers degeneration in part of sciatic nerve, although disclosed the trend to the reduction in their density (Fig. 3). In PRP, CBMA, and HAT groups, we registered no “free” neurolemmocytes. This fact can be the evidence of less damage to the nerve fibers after the MII; there were no clear morphologic signs of the nerve fibers’ progressive degeneration in terms from 5th day to 30th day. It only means that the demyelination and degeneration of isolated nerve fibers occurs on the MII level and distally away from the compressive ischemia level, in particular in tibial nerve.
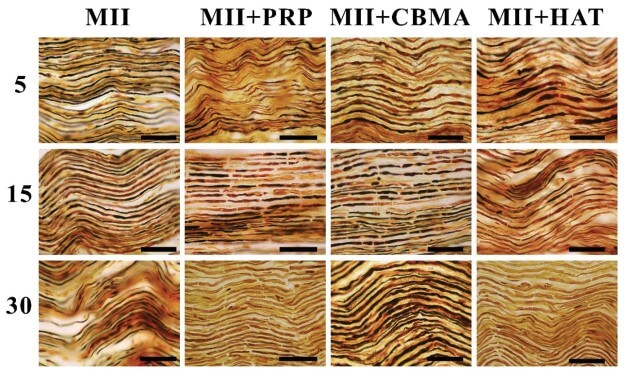
Fig. 1. A sciatic nerve of a rabbit on the 5th, 15th, and 30th day after the MII and the therapy. The impregnation with silver nitrate, scale bar 100 µm
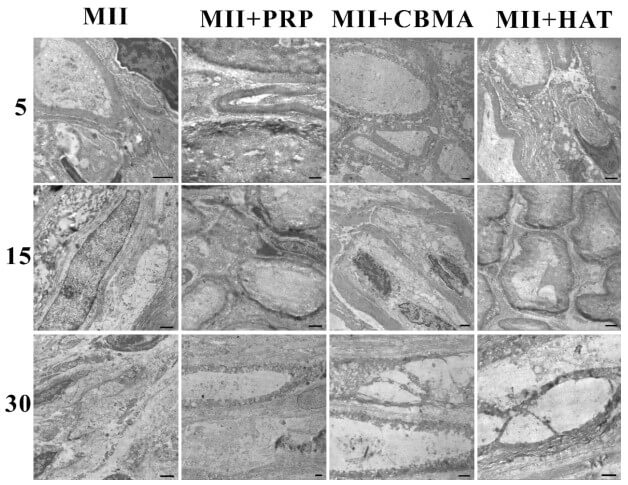
Fig. 2. A tibial nerve of a rabbit on the 5th, 15th, and 30th day after the MII and the therapy. electron microscopy, scale bar 1 µm
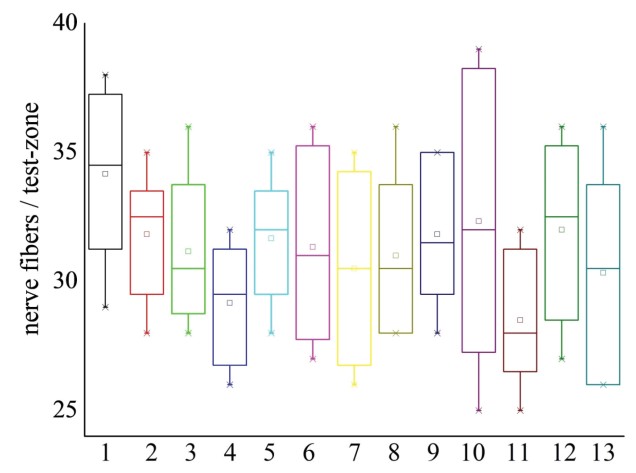
Fig. 3. The average amount of nerve fibers in the test-zones of a sciatic nerve after the MII and the injections of PRP, CBMA, and HAT note: 1 – control group; 2, 3, and 4 – MII on the 5th, 15th, and 30th day; 5, 6, and 7 – MII+PRP on the 5th, 15th, and 30th day; 8, 9, and 10 – MII+CBMA on the 5th, 15th and 30th day; 11, 12, and 13 – MII+HAT on the 5th, 15th, and 30th day
Analyzing the results of our own studies and the published articles, we concluded that muscles and peripheral nerves of a limb have different resistance against the compression ischemia. The skeletal muscles of a limb obviously endure significant damage from its compression. At the same time, the two factors, compression, and ischemia affect the limb’s muscles and nerves, but it is not always possible to distinguish between the morphologic consequences of both ischemic injury and compression ischemia, as the expressions of the cellular necrosis are non-specific.
Descriptions of damage to muscles, their dystrophy, and slight signs of regeneration appear already in publications. In the focus of our interest, we have another aspect of compression of a limb, namely peripheral nerve conditions. We assumed that large nerves of extremities, like a sciatic one, could avoid suffering from the necrosis thanks to their specific anatomical position in the limb. Perhaps, their deep anatomical placement in an intramuscular space of a limb can prevent severe compressive damage. We also investigated how PRP, CBMA, and HAT therapy can influence these changes.
The analysis of clinical cases showed that the loss of nerve fibers in chronic non-compression ischemia is not critical; it may be less than 5% of myelin fibers. The basic expressions of the disorder are disproportionally thin myelin sheath, demyelination of the fibers, myelin fibers deformities [13]. The reduced speed of nerve impulses is thus predetermined by an axonal degeneration rather than by a demyelination process [6]. However, spontaneous recovery is also possible. Thus, Hitoshi Nukada et al. have described regenerating nerve fibers in the ischemically damaged nerves of a limb after 1-6 months [13]. Damage to nerves and further nerve fibers degeneration can occur without a significant muscle injury [18]. This fact could be explained by the higher vulnerability of nerve fibers to ischemia, compared to the muscle tissue [12]. Thus, in critical ischemia of a lower limb, Kudykin M.N. et al. reported muscle hypotrophy in 40% of cases, but neuropathy – in 100% [7].
In an experimental paper, a ligature to an aorta does not cause total muscle ischemia due to the collateral blood flow; muscles and nerves suffer equally upon such conditions [3]. According to other data, in non-compression ischemic neuropathies, degenerative muscle changes could progress without significant necrosis of muscles, confirming the hypothesis that human distal nerve fibers, compared to muscle tissues, are more vulnerable to the acute noncompression ischemia of limbs [18]. After the limited microemboli of limb arteries, was reported the necrosis of small groups of muscle fibers, but muscles regenerate further [4]. In such cases, regeneration launches one month after the injury, while muscle fibrosis does not develop in non-compression ischemia.
Having analyzed the literature, we assumed that the extent of the nerve lesion depends directly on the value of compression of a limb by a tourniquet applied to the extremity. We came to this conclusion on the background of the absence of acute necrosis to structural elements of the sciatic nerve after 6 hours of the limb’s compression, while degeneration, destruction of nerve fibers’ myelin expressed brightly on the level of the tibial nerve, topographically equal to and distally from the compression area. The data described vary in different authors; this relates to the various methods used for study, like only electrophysiological or morphological. Thus, if the compression ischemia lasts more than 2 hours, the extent of degeneration progresses [3], while in other cases described, 12-hours compressing ischemia leads only to endoneurial edema [9]. Compression with a tourniquet on the level of 250 mm Hg leads only to a reversible nerve blockade, at 500 mm Hg – rarely to demyelination, while at 1000 mm Hg – to demyelination and mechanical damage to the nerve [3]. In the research, we determined the subfascial pressure, 30-70 mm Hg at the 3rd-6th hour of the MII. The discovered difference in the damage to the sciatic and tibial nerves indicates the difference in subfascial and intra-tissue pressures, reflected in more significant damage to a tibial nerve, due to its anatomic peculiarities placed more superficially, compared to the sciatic one.
During the first 4-6 hours after the tourniquet removal, the nerve conduction remained, while 8 hours later is did not restored [9]. Hence, on the background of these periods, one can say about a “therapeutic window” in compressive ischemia management. During the same time, we inserted PRP, CBMA, and HAT into the skeletal muscles of the shin. Histological and morphometrical studies showed neither positive nor negative results of the insertion of tissue suspension into the damaged muscles.
In all the samples of the nerves, we saw different types of damages: unequal thickness and myelin deformities, atypically thin fibers, degeneration of the axial cylinders, lamellar structures in neurolemmocytes, partial destruction of a nerve’s endoneurium. These changes were not all out; we have observed just a tendency to the decreased density of nerve fibers in the sciatic nerve on the 30th day after the MII, although the dystrophy of nerve fibers has already been noticed distally, in the tibial nerve. In other research, Richards R.L. described a significant increase in endoneurial collagen, progressing till complete replacement of the nerve bundles with collagen [5]. However, we have not discovered fibrosis in our own studies (except for the destruction of endoneurium and perineurium, isolated cell-less areas rich in collagen). We assessed all revealed disorders as the consequences of damage to the limb’s blood vessels, because the injuries to vessels (stasis, thrombosis, lytic cell death in vessels) occurred as in muscles’ myons, as in the capillaries of intramuscular space (unpublished data). However, the changes did not reach the total degeneration of the nerve. This fact could have several explanations. The blood supply to a limb’s peripheral nerve is multilevel, and the vessels cover reasonably the areas on different levels. Damage to a nerve due to a focal loss of perfusion has been considered before as highly improbable because collateral vessels ensure proper blood circulation in the nerve [5]. At the same time, the difference between vascularization and revascularization of a limb muscles exist. Thus, “fast muscles” (soleus) have a higher density of micro-vessels, more of them per muscle fiber, are longer, and with better VEGF expression, compared to the “slow muscles” (extensor digitorum longus). The explanation is in diverse physiology of various types of fibers in these muscles, their need for different oxygenation of the “fast” and “slow” aerobic and “fast” anaerobic fibers [15]. In our experiment, we neglected this division, as the compression ischemia was significant, non-selective, and led to the total necrosis of peripheral myons in all the muscles of a shin.
Consequently, the compression ischemia of a limb causes mild or moderate damage to the peripheral nerves of a limb, depending on the level of compression. Inserting PRP, CBMA, and HAT into the ischemic limb muscles showed no reliable effect on the development of structural changes in peripheral nerves while studying these processes in the ischemically damaged muscle fibers seems promising.
REFERENCES
1. Gaiovych I., Savosko S., Labunets I., Utko N., Makarenko A., Chaikovsky Y. Sciatic nerve regeneration after autografting and application of the bone marrow aspirate concentration.// Georgian Med News, 2019, 295, 145-152.
2. Halanski M.A., Morris M.R., Lee Harper B., Doro C. Intracompartmental Pressure Monitoring Using a Handheld Pressure Monitoring System.// JBJS essential surgical techniques, 2015, 5(1).
3. Harriman D.G. Ischaemia of peripheral nerve and muscle.// J ClinPatholSuppl (R CollPathol), 1977, 11, 94-104.
4. Hathaway P.W., Engel W.K., Zellweger H. Experimental myopathy after microarterial embolization: Comparison with childhood X-linked pseudohypertrophic musculardystrophy.// Archives of Neurology, 1970, 22, 365-378.
5. Richards R.L. Ischaemic lesions of peripheral nerves: a review.// J Neurol Neurosurg Psychiatry, 1951, 14(2), 76-87.
6. Kaku D.A., Malamut R.I., Frey D.J., Parry G.J. Conduction block as a nearly sign of reversible injury in ischemic monomelic neuropathy.// Neurology, 1993, 43(6), 1126-1130.
7. Kudykin M.N., Sheiko G.E., Belova A.N. Peripheral neuropathy in critical limb ischemia.//RMJ, 2018, 6(II), 70-73.
8. Liu Z.Y., Chen Z.B., Chen J.H. A novel chronic nerve compression model in the rat./ Neural Regen Res, 2018, 13(8), 1477-1485.
9. Lundborg G. Limb ischemia and nerve injury.//ArchSurg, 1972, 104(5), 631-632.
10. Malahias M.A., Chytas D., Babis G.C., Nikolaou V.S. Platelet-rich plasma guided injections: clinical application in peripheral neuropathies.//FrontSurg. 2014, 1, 41.
11. Muthuraman A., Ramesh M., Sood S. Development of animal model for vasculatic neuropathy: Induction by ischemicreperfusion in the rat femoral artery.// Journal of Neuroscience Methods, 2010, 186(2), 215-221.
12. Nukada H. Ischemic neuropathy.// Rinsho Shinkeigaku, 1990, 30(12), 1368-1370.
13. Nukada H., van Rij A.M., Packer S.G.K., McMorran P.D. Pathology of acute and chronic ischaemic neuropathy in atherosclerotic peripheral vascular disease. //Brain, 1996, 119(5), 1449-1450.
14. Park H.T., Kim J.K., Tricaud N. The conceptual introduction of the “demyelinating Schwanncell” in peripheral demyelinating neuropathies.//Glia,2019, 67(4), 571-581.
15. Ranjbar K., Fayazi B. Vascularisation of Skeletal Muscle. In: Muscle Cells — Recent Advances and Future Perspectives. Ed. by Mani T. Valarmathi. Intech Open, 2019, 180.
16. Sorokin Yu.N., Sagaradze S.A., Melnikov A.V. Acute Ischemic Neuropathy.//International Neurological Journal [S.l.], 2014, 2(64), 100-105.
17. Ugalde V., Rosen BS. Ischemic peripheral neuropathy.//Phys Med RehabilClin N Am, 2001, 12(2), 365-380.
18. Wilbourn A.J., Furlan A.J., Hulley W., Ruschhaupt W. Ischemic monomelic neuropathy.//Neurology, 1983, 33(4), 447-451.